© 2023, Randy Mosher / Craft Beer & Brewing Magazine
Beer is more complex than any other beverage known. No one’s keeping the master list of odor chemicals, but it’s huge. Hops alone contain more than 1000 terpenoids with citrus, floral and other aromas, with many other chemicals, too. In malt, Maillard and other browning processes create hundreds more. Fermentation and subsequent maturation creates a third enormous family of aromas, yet there are more. Add them all up and you get far in excess of the widely quoted number of 600–1000 odor chemicals in wine.
Without the comforting framework of styles, it can be a little hard figuring out where to focus one’s attention. Clearly, we’re not equipped to pick out a thousand or more aroma chemicals from even the most thoughtful nosing of a beer. Here’s the question: how does our olfactory system make sense of such complex stimuli without overwhelming our neural resources?

I came across a fascinating article on wine that really promises to upend the way we think about aromatically complex subjects. To make sense of their research, we have to back up a couple of steps and consider how, exactly, we smell. This crucial and mysterious sense is fed by an array of close to 400 different olfactory receptor (OR) types, of which we have varying numbers of copies. While there are exceptions, most receptors are stimulated by more than a single chemical, while most chemicals stimulate more that one receptor type. These responses may be positive, negative or null, and the responses for each OR and chemical vary by concentration. For complex reasons, there is also quite a bit of interaction between neighboring ORs, which tend to be responsive to similar chemicals, as well as competition between odorant chemicals for receptors.
Any smell creates a specific pattern of olfactory receptor neuron responses. When this pattern is fed to the brain through the olfactory bulb, it is translated from a pattern of responses to chemistry to a pattern of meaning. It is this pattern the brain actually recognizes from memory and ultimately uses to make decisions.
How the brain does this is still a deep mystery, but one of the key features is that the chemical coding the nose initially generates is utterly and irretrievably lost when it hits the olfactory cortex in the brain. Scientists call this configural processing, meaning that individual sensory input comes together to create a percept, or sensory experience that is different from its components, and which is not readily broken back down into them. Smell behaves more like this than any other sense. These configural odors are everywhere in our lives whether we realize it or not.
The best example I can think of is that of cola. This ubiquitous soft-drink flavor is composed of a number of terpenoid-rich botanicals including lemon, lime, orange peel and blossom, coriander, cinnamon and nutmeg. Combine them in the right proportion and cola magically appears to tickle your nose. While those who work with this everyday might be able to tease out the threads, I defy any mere mortal to do so. Cola is not the simple sum of its parts.
One of the more revealing ways to study the smells of foods and beverages is to analyze their aroma constituents as well as their relative intensities compared to their known detection thresholds. For each compound, this results in an odor activity value (OAV). An OAV of one is where the threshold meets the quantity present, meaning it’s probably detectable and therefore relevant to the overall odor.
To study a complex mix like wine, these values are used to create a “model” wine. Single chemicals are blended according to the original analysis, which is then compared to the original. If it’s the same, the next step is to start removing things to find which of the aroma chemicals really matter. A typical wine may contain hundreds of aroma chemicals, but typically only a couple of dozen are needed to create a convincing facsimile. In this study of fruity esters in wine, researchers included 14 ethyl esters, only six of which had an OAV greater than one. What they found was remarkable. They were able to remove every ester but one and still maintain the same fruity character as long as they bumped up the quantity to match the original intensity. Amazingly, this took five times as much of the replacement ester in OAV terms as the original components. As a group, the esters were so resilient that scientists termed them a “buffer.” Even though many of the original esters were far below threshold values, they were strongly interacting—demonstrating superadditivity, a kind of synergism.
Nor was this the only type of nonlinearity. They discovered destructive interactions between desirable aromas like fruitiness and problem odors like smoke, green pepper, barnyard (Brettanomyces) and mold, which is a well-known aroma wrecker in many situations. These interactions give wine off-flavors potency far beyond their own aroma intensities, and the same happens in beer as well.
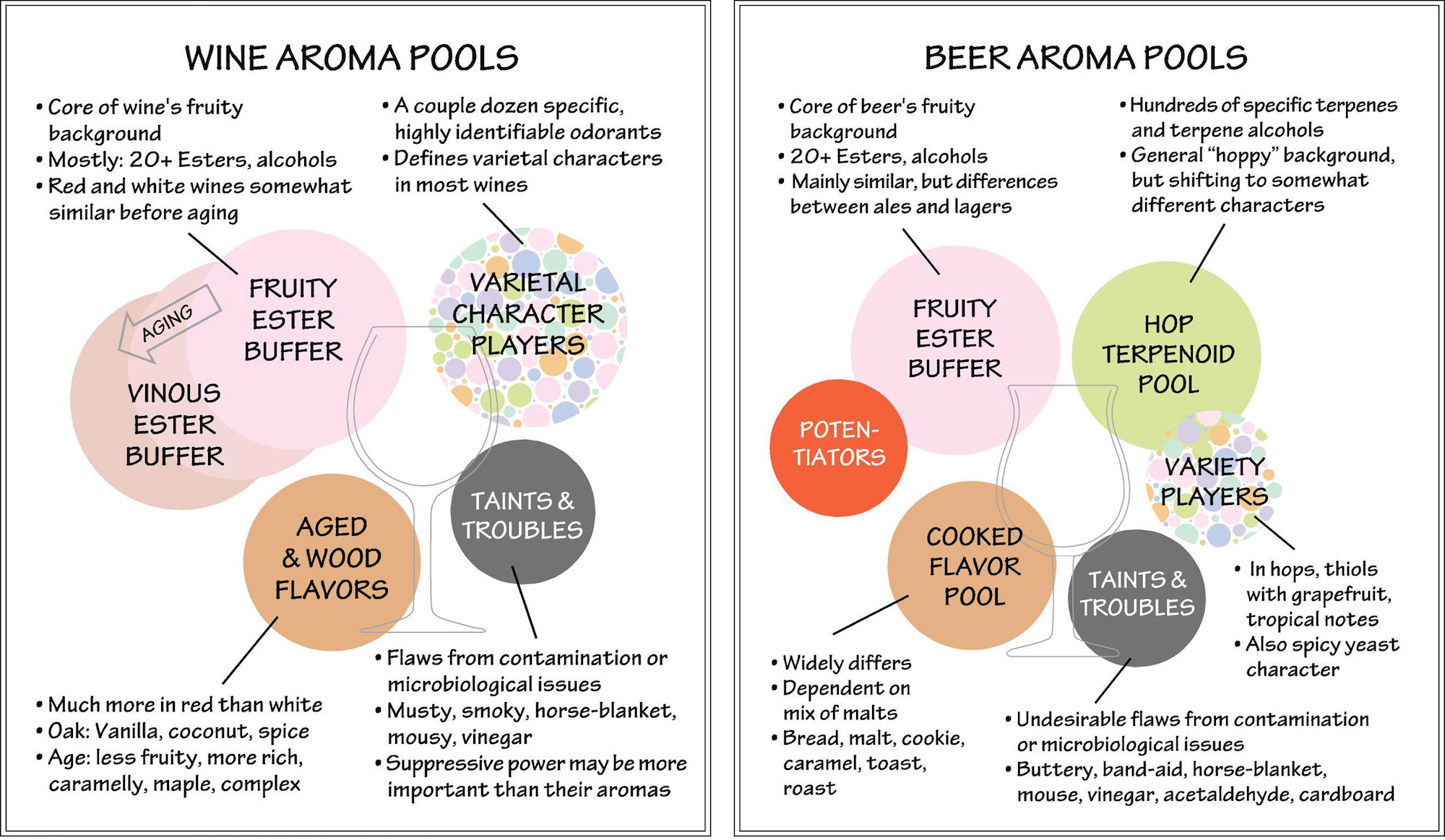
To sum up, wine has a pool of self-amplifying fruity odors and a second pool of mostly spice and cooked aromas derived from wood, especially in reds. An unruly set of varietal aromas are different enough from fruity esters to stand out from them, although some can simultaneously enhance fruitiness. Finally there are bad actors sowing destructive mayhem.
Is beer odor structured similarly? It seems like it may be. Since the fruity esters in both wine and beer derive from yeast—shockingly, wine gets little of its fruitiness directly from grapes—each has a similar fermentation flavor pool. Both contain a grab-bag of varietal impact compounds. While wine has a pinch of cooked flavors originating from toasted wood, it’s nothing like the scope and centrality of the cooked flavors in beer, which originate from kilning and other heat-processing of grains like malt. This one obviously varies widely by recipe, but is always a strong presence. It’s also known that many Maillard components, especially at the light and malty end of the spectrum, are capable of enhancing other aromas and even sweet taste in products as diverse as cheddar cheese, cereal, tomatoes and wine. A chemical called maltol is said to be able to enhance just about anything. However, these same compounds can be potent maskers of bright, fruity flavors and contribute to beer staling.
Then, there are the hops with their vast pool of terpenoids. In wine, a handful of terpenes are relevant to varietal character, especially in so-called “aromatic” whites,” but in beer they are a main attraction. These are difficult chemicals to get a grip on in terms of aromas. While generally floral and citrusy, we generally don’t encounter them by themselves, although with some practice we can learn to identify a few of the more abundant ones: geraniol (rose, geranium), linalool (coriander, lavender) and beta-citronellol (lemongrass, citronella), but there are many others in the mix. The result is beer’s generally “hoppy” background.
So far, there’s nothing so dramatic in the beer literature as the wine re-blending experiment above, but there is evidence for reinforcing interactions between closely related terpenoids such as geraniol, linalool and beta citronellol. There are also known interactions between different classes of hop aroma compounds, including a character-bending effect between monoterpene alcohols such as linalool and branched chain fatty acids such as isovaleric acid that may be responsible for melon character in some hops. It’s well known that hop thiols, in addition to having their own distinctive aromas, are strongly reinforcing of each other as well as of terpenoids and other odor chemicals. Asked if she thought there was a terpenoid pool in beer similar to the ester buffer in wine, Head of Innovation and Education for the hop giant, BarthHaas, Dr. Christina Schoenberger said “Absolutely, I would support that.”
And it’s no secret among creative brewers of IPAs that there are a lot of synergies between hop varieties that help them punch above their weight. Unfortunately, it’s difficult to come up with any generalities about this approach.

From a standpoint of a flavor creator, there are a lot of helpful concepts here. First, an ingredient need not be individually distinguishable to have a meaningful effect. Therefore, a number of small contributions may add up to something much more impactful than would be expected. Second, these “buffer” pools are very useful in establishing the overall character of a product, but each constitutes a crowd with a mind of it’s own. Sometimes you can manage to shove them one way or another just a bit, but a smarter strategy is to use contrasting things that can rise above them or enhance certain aspects of them. It’s futile to try to really control them in the naturally fermented products we’re talking about here.
The group of variety players includes positive odors such as hop thiols with their valued grapefruit and tropical notes, but some can have toasted onion/garlic notes. Others can cut both ways. The norisoprenoid beta-damascenone is potently fruity, but its cooked apple and dried fruit can be a stale note in hoppy beers.
As in wine, the taints & troubles pool is highly destructive to positive beer flavor and not just because of their own unpleasant smells. Most are known maskers of fresh, fruity and hoppy aromas.
As tasters, we love to be able to dissect aromas down to the molecular level, but it’s clear that this is often an unworkable strategy, especially with positive flavors. Sometimes it’s better to step back and bit and contemplate the mysteries and magic contained in the depths of the flavor pools.